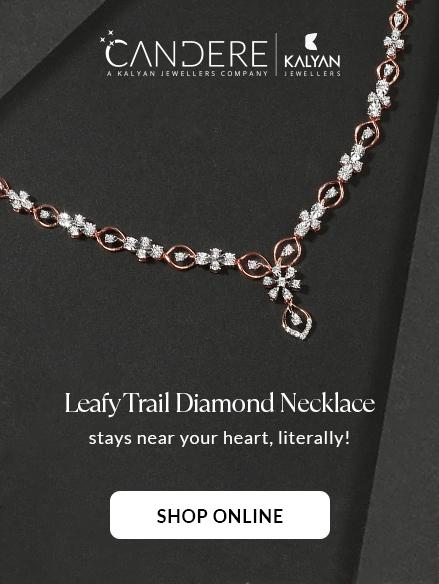
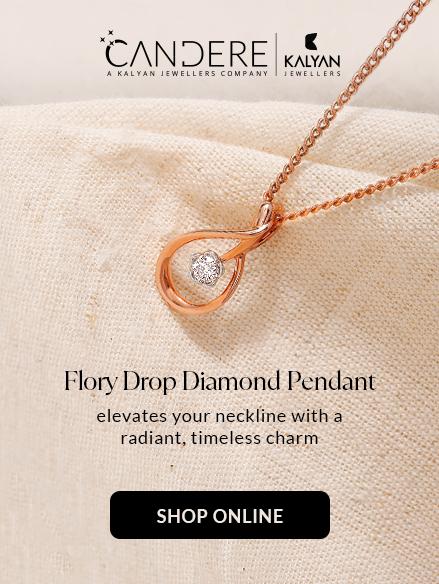
E-Store
Now you can find all your favourite collections online! Shop from the comfort of your homes at Candere, Kalyan Jewellers official e-commerce store.
Brands Family
India’s beauty lies in its diversity. Every part of India has its unique jewellery, the expertise to create those exist only in that region, passed on through generations. Kalyan’s biggest strength is in these craftsmen who create unique designs just for us from every nook and corner of India. And this range of jewellery, with those subtle nuances and the perfect finish which you won’t find anywhere else is what we bring you through our various brands.